18 Mar A quick guide to COVID-19 testing
In early March, an El País article expressed that although the diagnostic test for COVID-19 itself can be completed in 4 hours, the entire process from sample collection to delivery of results can take days. So, what’s being done to test for the novel coronavirus? Here’s a quick guide to help you better understand the process of COVID-19 testing!
What samples are taken for COVID-19 tests?
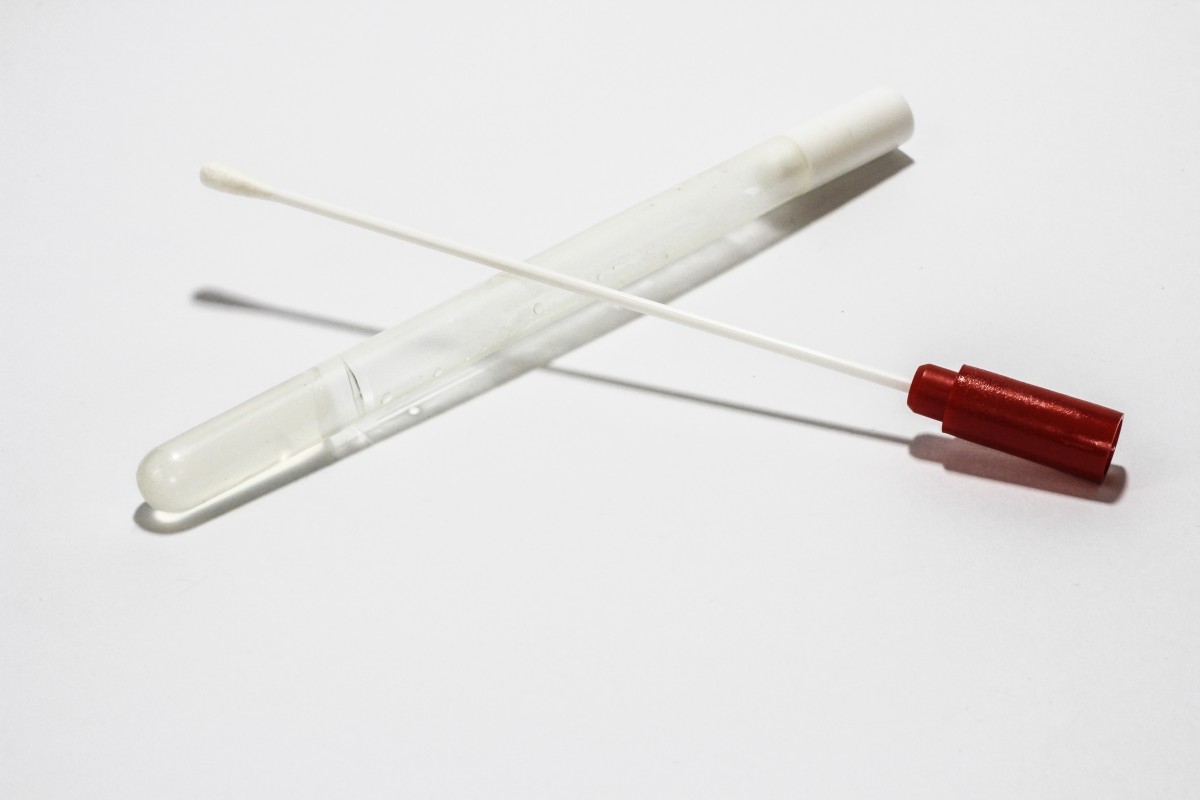
Experts with the World Health Organisation note that virus shedding patterns aren’t yet well understood. Whereas respiratory samples have the greatest yield, it is true that the virus can be detected in other specimen, such as stool and blood.
Current guidelines advise that upper respiratory specimen (nasopharyngeal swabs and oropharyngeal swabs) and/or lower respiratory specimen (sputum, endotracheal aspirate or bronchoalveolar lavage) should be collected, at minimum, for testing if diagnostic criteria is met.
What is the testing method for COVID-19?

Screening is done on the previously mentioned respiratory samples. Interim Guidance from the World Health Organisation states that suspect cases should be screened for the novel coronavirus with nucleic acid amplification tests (NAAT). What does ‘nucleic acid amplification test’ mean? Thankfully, its name is self-explanatory!
Fakruddin and colleagues state that polymerase chain reaction (PCR) was the first nucleic acid amplification method. According to an American Society for Microbiology publication, these methods are classified according to the source of the nucleic acid that’s amplified in the procedure.
Target amplification methods, a classification of NAAT, use enzymatic tools to increase the concentration of target nucleic acids in the sample. PCR is a target amplification method. We can use it to replicate and detect DNA.
SARS-CoV-2, the virus that causes the disease COVID-19, is an RNA virus. To detect its genetic material, the RNA has to be converted to cDNA using a reverse transcriptase (RT) enzyme before amplification by PCR. This is known as RT-PCR, and it is also a target amplification method. Current targets for COVID-19 diagnosis include the N, E, S and RdRP genes of SARS-CoV-2.
Is RT-PCR the only way to test for COVID-19?
The World Health Organisation interim guidance lists two other methods besides NAAT that can aid COVID-19 investigations. These are serological testing, and viral sequencing.
Serological testing, according to the American Centers for Disease Control and Prevention, looks for the presence of antibodies – specific proteins made in response to infections. They are found after infection and their presence indicates that a person had an immune response to SARS-CoV-2. Viral sequencing, which also has a rather self-explanatory name, is used to confirm the presence of the virus.
Serological testing and viral sequencing alone cannot be used to diagnose COVID-19 because current guidance calls for the use of NAAT. Regardless, they are still useful investigative tools.
Serological testing lets researchers assess the attack rate or extent of an outbreak retrospectively. Viral sequencing can be useful in monitoring for viral genome mutations that could influence how well medical measures, like diagnostic tests, work.
We’re all contributing to the fight against this pandemic. Follow us on Twitter / LinkedIn for updates!


Sorry, the comment form is closed at this time.