29 May Transfection
Transfection
Transfection is a process in which molecules like DNA, RNA, proteins and oligonucleotides are deliberately introduced into the recipient cell, integrating into the chromosomal DNA of the recipient cell causing a change in the properties of the cell. In this article, we will discuss the methods of transfection, stable vs transient transfection, and the four most relevant types to perform transfection.
Methods of transfection
- Transient transfection
- Stable transfection
- Transient Transfection
Cells that are transfected transiently express the foreign gene but do not get integrated into the genome. As a result, no replication takes place. Consequently, the foreign gene is expressed in the cell for a certain finite period of time and then is lost through cell division or other factors.
- Stable Transfection
To undergo a stable transfection, the procedure starts with a transient transfection followed by many other infrequent but very important procedures. In a small proportion of transfected cells, the foreign gene becomes a part of the genome and gets replicated. Successors of this transfected cells will also have this foreign gene incorporated in their genomes.
Has the foreign gene been transfected?
Now, the question that arises is how do we know the foreign gene has been transfected in the cell when there is no replication taking place? First of all, we add a reporter gene to the complex so we get to know whether the foreign gene has been successfully transfected in two to three days.
Also, to distinguish between stable and transient transfected cells, the researchers add selectable markers and verify the different types. Normally, what they do is add antibiotic markers to the cells which they transfect transiently and stably in such a way that the stable cells are resistant to the antibiotic and transient cells are not resistant to the antibiotic. Hence, only the stable transfected cells are going to last for longer in the cell and replicated in future generations
- Stable vs Transient transfection. Which one should you use?
Transient transfections are preferred in experiments that are short term and transient transfection is often used for studying the effects of short-term expression of gene or gene products or small-scale protein production. On the contrary, stable transfection is done for long – term and prolonged experiments for example for long term protein production, preparation of drugs or pharmaceutical industry, gene regulation or gene therapy.
Also making stable cell lines has become a tedious job, making cell lines via transient and stable transfected cells is much easier and faster.
Now, these two different types of transfections can be done in four different ways. They include Calcium phosphate method, Electroporation, Viral Methods and Lipofection. Our main focus is on Lipofection as for now we sell only the Lipofection product named as Lipotransfectin.
-
Calcium Phosphate method
It is a widely used method for introducing foreign DNA plasmids into cells. The method was first described in 1973 by Graham and Van der Ebb. The calcium phosphate transfection technique involves the precipitation of DNA and Calcium phosphate allowing the formation of small complexes that get internalised into a cell and eventually gets transfected.
Advantages and Disadvantages
The cost- efficiency and simplicity of the method allows its use in many different cultures, this method can accommodate a high concentration of DNA. One of the drawbacks is that the Calcium Phosphate method has only 10% efficiency. Also, sometimes the calcium phosphate can disrupt the cell membrane and cause cell death. Thus, it is not suitable for protocols that require a long incubation period.
-
Electroporation
A method used to apply an electric current across the cell membrane of a particular cell which leads to the formation of a temporary pore. This “pore” helps in the transfer of exogenous materials from the medium to the cytoplasm or nucleus and thus transfecting the cell. Some of the advantages of Electroporation include its versatility of being effective with almost all the different cell types.
Advantages and Disadvantages
The amount of DNA required for electroporation is much lesser than the other procedures. The disadvantages include cell damage which occurs if the pulses are of the wrong length or intensity which can cause a larger pore than needed or a smaller pore which would lead to cell rupture. Also, the transport of material in and out of the cell is non-specific and can lead to ion imbalance and ultimately cell rupture.
-
Viral methods
The viral method uses viral vectors to deliver nucleic acids into cells. The different types of viral vectors that can be used include Retroviruses like Human immunodeficiency virus (HIV) and Murine leukaemia virus (MuLV) and DNA viruses like Herpes Simplex virus (HSV), Adenovirus and Adeno-associated virus (AAV).
Advantages and Disadvantages
The advantages of using the viral methods include the simplicity and effortlessness of the viral infection, works well in vivo and also in vitro and the high gene delivery efficiency which has an amazing range of 95-100%. The viral method, unlike the other methods, seems like it has more disadvantages than advantages. It is a labour intensive task which not only requires performing the task manually but also keeping a close watch on the task. Also, it requires many approvals from various esteemed institutions and review boards, P2 containment required for most of the viruses, most of the viruses are lytic and require packaging of cell lines. As a result, the best time to use this method is for introducing a single cloned gene only that needs to be highly expressed.
-
Lipofection
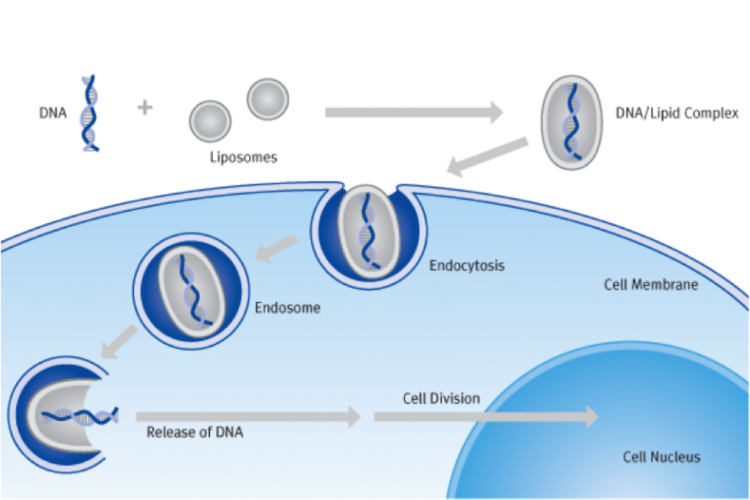
Lipofection refers to the method where a lipid (mostly cationic) is used to absorb the nucleic acid into the cell, the genetic material is transferred into the cell with the help of liposomes. Liposomes are vesicles or components that can fuse with the cell membrane. The following complex formed is referred to as Lipoplex. The advantages of lipofection include high efficiency, ability to transfect different types of nucleic acid in a broad range of cell types, reproducibility, easy to handle and use,
Advantages and Disadvantages
The advantages of lipofection include high efficiency, ability to transfect different types of nucleic acid in a broad range of cell types, reproducibility, easy to handle and use, the ability to deliver DNA, RNA and proteins, low toxicity. The main disadvantage is its total dependence on the cell type and culture medium efficiency, it demands optimised conditions of transfection reagents and culture mediums.
Lipotransfectin- Our customer’s favourite product
Transfection agent for mammalian cells
Figure 2: Lipotransfectin from Solmeglas.
General Information:
Lipofection is a state-of-the-art transfection agent for exclusive use in mammalian cell research. Its novel composition combines RMA (Repulsive Membrane Acidolysis) technology with a new Toxic Optimised module (TOP-technologies). It regulates the release of genetic material (DNA and RNA), reducing toxicity and increasing the efficiency of transfection.
General Information:
Reference Product Presentation
SBM0959 Lipotransfectin 1 ml
Specifications:
| Applications | Transfection of nucleotides in mammalian cells. |
| Formulation | Cationic lipids + co lipids in water. |
| Assays | Up to 1000 (24 wells) or up to 300 (6 wells) with 1ml of the agent.
|
| Sterility | Tested. |
| Cell culture | Tested. |
| Shelf life | 1 year. |
| Storage | 4 ºC. |
| Long term storage | -15ºC |
Quality Assurance:
Standard assays for transfection. Verified absence of fungi and batteries using medium thioglycolate.
Technical specifications of the supply:
Lipotransfectin guarantees high transfection efficiency for a large number of cell lines. The following is a table with some of the most used cell lines and the mean of their minimum transfection efficiency percentages. Our company Solmeglas has evidence for this table.
| Cell lines | Transfection efficiency (%) |
| 293-H, HEK293, HEK 293T | 99% |
| CHO, CHO-S, CHO-K1 | 90% |
| COS-7, COS-7L | 60% |
| BE (2)C, BE(2)M17 | 53% |
| MDCK | 60% |
| HTI1080 | 75% |
| HeLa | 94% |
| PC12 | 30% |
Explanatory remarks:
1- Considering that the most commonly used DNA amounts for 24 and 6-well plates are usually 0.5 μL to 2 μL. Thus, with the 1.5 ml format, it would be more than sufficient to perform 750 to 1000 transfections in the 24-well plates and 190 to 300 in the 6-well plates.
2 – Lipotransfectin is used with a much simpler protocol compared to other similar agents of other brands. It can be used with presence or absence of serum in the culture medium. The effectiveness of the product remains unaffected.
3- It also allows to perform automated applications «High throughput» type in 9-well plates.
Storage:
Lipotransfectin will be provided to the user at an ambient temperature. It is advisable to store the product at -15 ºC to avoid the natural ageing process of the liposomes. There is no evidence of degradation of the product by successive freezing and thawing.
References
- Potter, H., & Heller, R. (2011). Transfection by electroporation.Current Protocols in Neuroscience, A-1E.
- https://es.slideshare.net/SachinMehta12/electroporation
- Pfeifer, A., & Verma, I. M. (2001). Gene therapy: promises and problems.Annual review of genomics and human genetics, 2(1), 177-211.
- http://www.bio-rad.com/webroot/web/pdf/lsr/literature/10-0826_transfection_tutorial_interactive.pdf


Sorry, the comment form is closed at this time.